| Entrance | Mainstreet | Wiki | Register |
|
# of watchers: 1
|
Fans: 0
| D20: 13 |
| Wiki-page rating |  Stumble! Stumble! |
| Informative: | 0 |
| Artistic: | 0 |
| Funny-rating: | 0 |
| Friendly: | 0 |



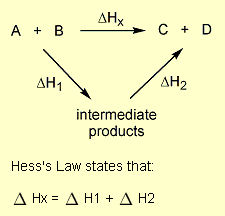

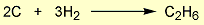
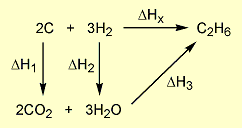
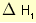 = 2 × enthalpy of combustion of carbon (2 × -394).
= 2 × enthalpy of combustion of carbon (2 × -394).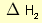 = 3 × enthalpy of combustion of hydrogen (3 × -286).
= 3 × enthalpy of combustion of hydrogen (3 × -286).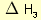 = reverse of enthalpy of combustion of ethane. The enthalpy of combustion of ethane has a negative value so the reverse will have a positive value (+1560).
= reverse of enthalpy of combustion of ethane. The enthalpy of combustion of ethane has a negative value so the reverse will have a positive value (+1560).



2005-09-28 [Firenze]: Thank you I spent a while making this page
2005-11-28 [japegrin]: how does one go about becoming a student?
| Show these comments on your site |
|
Elftown - Wiki, forums, community and friendship.
|